VONVENDI Dosing for 3 Indications1
Indications
VONVENDI [von Willebrand factor (recombinant)] is indicated in adult and pediatric patients with von Willebrand disease (VWD) for1:
- On-demand treatment and control of bleeding episodes.
- Perioperative management of bleeding.
For adult patients only:
- Routine prophylaxis to reduce the frequency of bleeding episodes.
Your adult patient's prophylaxis journey begins with a straightforward dosing approach1
Your patients’ journey begins with a single starting dose with a single factor1
For routine prophylaxis, administer: 40 to 60 IU/kg of VONVENDI twice weekly for initiation of prophylactic treatment1
- Treat breakthrough bleeding as per the dosing guidelines for on-demand administration1
Consider self-infusion with VONVENDI for your patients with VWD
VONVENDI for on-demand treatment may be dosed with or without rFVIII1*
VONVENDI enables healthcare providers to manage von Willebrand factor (VWF) and FVIII levels separately and specifically, based on patient need1†‡
For the first dose, administer VONVENDI alone if FVIII:C level is ≥40% or if an immediate rise in FVIII:C is not necessary.1
*VONVENDI contains only trace amounts of rFVIII.1
†If rFVIII is administered, see rFVIII package insert for reconstitution and administration instructions.1
‡A bleed could be considered major if red blood cell transfusion is either required or potentially indicated or if bleeding occurs in a critical anatomical site (eg, intracranial or gastrointestinal hemorrhage).1
Administer VONVENDI with rFVIII if the FVIII:C level is less than 40%, or is unknown, to control bleeding. The rFVIII dose should be calculated according to the difference between the patient’s baseline plasma FVIII:C level, and the desired peak FVIII:C level to achieve an appropriate plasma FVIII:C level based on the approximate mean recovery of 2 (IU/dL)/(IU/kg). Administer the complete dose of VONVENDI followed by rFVIII within 10 minutes.1
Learn about the endogenous rise in FVIII levels following VONVENDI infusion.
Consider prophylaxis for your adult VWD patients
VONVENDI for management of surgical bleeding may be dosed with or without rFVIII1*
Perioperative dosing of VONVENDI for elective surgery†

12-24 hours prior to surgery1:
VONVENDI may be administered to allow endogenous FVIII levels to increase

Within 3 hours prior to surgery1:
- Assess FVIII:C to ensure minimum target levels are achieved
- Administer VONVENDI within 1 hour prior to surgery with or without rFVIII
- Administer VONVENDI alone if FVIII:C is at or greater than minimum target levels
- Administer VONVENDI with rFVIII if FVIII:C is below minimum target levels
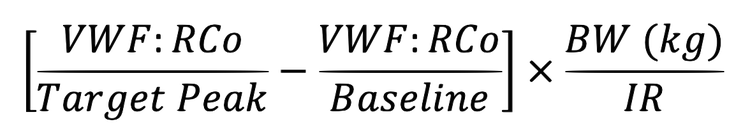
After surgery1
- Monitor VWF:RCo and FVIII:C plasma levels starting at 12-24 hours and at least every 24 hours
- Dose VONVENDI with or without rFVIII based on patient need. If necessary, the frequency of VONVENDI dosing should range between twice a day and every 48 hours
Dosing of VONVENDI, with or without rFVIII, should be based on patient need as determined by monitoring levels and clinical judgment.1
*VONVENDI contains only trace amounts of rFVIII.1
†Please see Full Prescribing Information for VONVENDI dosing in the emergency setting.1
‡IR=Incremental Recovery as measured in the subject. If the IR is not available, assume an IR of 2.0% per IU/kg.1
§Additional rFVIII may be required to attain the recommended FVIII:C target peak plasma levels. Dosing guidance should be based on the IR.1
Be a VONVENDI Prophylactivist
VONVENDI is the first and only recombinant VWF for prophylaxis1-5
Support for Your Patients
Browse a variety of resources from Takeda for your appropriate patients
Contact a Takeda Rep
Get in touch with a Takeda representative in your area
References
- VONVENDI [von Willebrand factor (Recombinant)] Prescribing Information.
- HUMATE-P [Antihemophilic Factor/von Willebrand Factor Complex (Human)] Prescribing Information. CSL Behring GmbH; 2020.
- WILATE Full Prescribing Information. Paramus, NJ: Octapharma; rev December 2023.
- ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) Prescribing Information. Grifols.
- FDA Approves Prophylactic Treatment with VONVENDI [von Willebrand Factor (Recombinant)] for Adult Patients Living with Severe Type 3 von Willebrand Disease (VWD). Businesswire. Accessed August 14, 2025. https://www.businesswire.com/news/home/20220131005214/en/FDA-Approves-Prophylactic-Treatment-with-VONVENDI-von-Willebrand-Factor-Recombinant-for-Adult-Patients-Living-with-Severe-Type-3-von-Willebrand-Disease-VWD



