Plan for surgical procedures with adults with vwd1
Indications
VONVENDI [von Willebrand factor (recombinant)] is indicated in adult and pediatric patients with von Willebrand disease (VWD) for1:
- On-demand treatment and control of bleeding episodes.
- Perioperative management of bleeding
For adult patients only:
- Routine prophylaxis to reduce the frequency of bleeding episodes.
100% overall hemostatic efficacy for both major and minor surgeries in adults in a pivotal surgical trial1
All 15 participants treated with VONVENDI with or without rFVIII had hemostatic efficacy ratings of “Excellent” or “Good”1
Overall hemostatic efficacy (rated by the investigator)1
100% (15/15) for major and minor surgeries (90% CI, 81.9-100)

Intraoperative hemostatic efficacy (rated by the surgeon)1
100% (15/15) for major and minor surgeries (90% CI, 81.9-100)

- Patients with VWD experienced a normal amount of blood loss2
- Predicted blood loss in hemostatically normal patients (average [SD]): 106 mL (162) [n=14])
- Actual blood loss in patients treated with VONVENDI (average [SD]): 94 mL (178 [n=15])

Study description
Hemostatic efficacy of VONVENDI was evaluated in a prospective, open-label, multicenter trial in adults with severe VWD undergoing elective surgery. All 15 subjects who completed the study received a preoperative dose of 40–60 IU/kg VONVENDI 12–24 hours before surgery to raise FVIII to target levels. Within 3 hours prior to surgery, FVlll:C was assessed to confirm ≥30 IU/dL for minor and ≥60 IU/dL for major surgeries. Within 1 hour prior, VONVENDI was administered, with rFVIII added as needed. Overall hemostatic efficacy (the primary endpoint) was assessed 24 hours after last perioperative VONVENDI infusion or at completion of study, whichever occurred earlier, using a 4-point scale (Excellent=l, Good=2, Moderate=3, None=4).1
Primary endpoint
- Overall hemostatic efficacy assessed by the investigator 24 hours after last perioperative VONVENDI infusion or at completion of day 14 visit, whichever occurred earlier1
Secondary endpoints included1,2:
- Intraoperative hemostatic efficacy as assessed by the operating surgeon
- Intraoperative actual blood loss relative to predicted blood loss
Efficacy was defined as a mean rating score of ≤2, assessed using a 4-point rating scale (Excellent=1, Good=2, Moderate=3, None=4).1
Excellent: Hemostasis achieved with VONVENDI, with or without recombinant factor VIII (rFVIII), was as good or better than expected for a hemostatically normal patient undergoing the same type of surgery.1
Good: Hemostasis achieved with VONVENDI, with or without rFVIII, was probably as good as expected for a hemostatically normal patient undergoing the same type of surgery.1
Study safety
TEAEs were reported in 6 patients. 11 of 12 adverse events were considered unrelated to treatment: acne, anemia, deep vein thrombosis (DVT), diverticulitis, dizziness, dry skin, headache, joint swelling, nasopharyngitis, pelvic pain, and peripheral swelling. One DVT event, considered possibly related to VONVENDI, was managed throughout the postoperative period; it subsequently resolved.2
VONVENDI was all most adult patients needed to manage surgical bleeding*
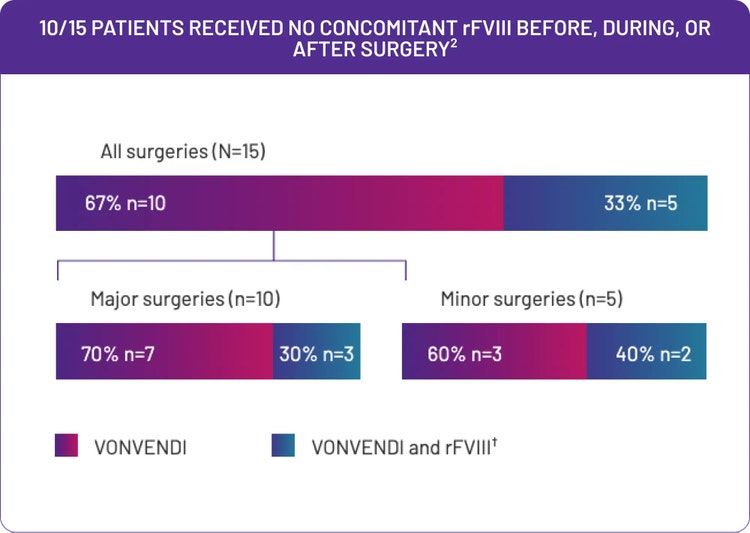
1 dose per day was all most patients needed for postoperative bleeding management.‡ 13/15 patients in the trial received VONVENDI postoperatively2§
*VONVENDI contains only trace amounts of rFVIII.1
†Of the 5 patients who did receive concomitant rFVIII, 2 patients received a preoperative infusion (1 infusion each), 1 patient received an intraoperative infusion, 1 patient received a postoperative infusion, and 1 patient received both 1 preoperative and 6 postoperative infusions. Only 2 out of a total of 11 infusions in these 5 patients were performed when the FVIII:C level was actually below a protocol-defined target level.2
‡Three patients undergoing major surgery required more frequent dosing of VONVENDI.2
§Two patients undergoing minor surgery did not require any postoperative infusions.2

VONVENDI surgical adverse events2
Treatment-emergent adverse events were reported in 6 patients
11 of 12 adverse events were considered unrelated to treatment: acne, anemia, deep vein thrombosis (DVT), diverticulitis, dizziness, dry skin, headache, joint swelling, nasopharyngitis, pelvic pain, and peripheral swelling.
One DVT event considered possibly related to VONVENDI was managed throughout the postoperative period; it subsequently resolved.
Review Safety Data
See the overall safety profile and adverse events for VONVENDI
Read Dosing by Indication
Prophylaxis, on-demand, and perioperative dosing for VONVENDI1
Contact a Takeda Rep
Get in touch with a Takeda representative in your area
References
- VONVENDI [von Willebrand factor (Recombinant)] Prescribing Information.
- Peyvandi F, Mamaev A, Wang JD, et al. Phase 3 study of recombinant von Willebrand factor in patients with severe von Willebrand disease who are undergoing elective surgery. J Thromb Haemost. 2019;17(1):52-62.